30+ Calculating Equivalence Point
Determine the pH of resultant solution when 250 cm 3 of 01 moldm-3 NaOH. Web Video Quiz Course 61K views How to Find the Equivalence Point Learning how to find the equivalence point is an important part of many chemistry classes.

Titration Curves Equivalence Point Article Khan Academy
Web Youll get a detailed solution from a subject matter expert that helps you learn core concepts.
. Web This problem has been solved. Web Before the first equivalence point the pH is controlled by a buffer of H 2 A and HA. Calculate the pKa and thus Ka of.
Web A titration curve is a plot of the concentration of the analyte at a given point in the experiment usually pH in an acid-base titration vs. Web Using Conductivity to Find an Equivalence Point. Web the goal is to determine the equivalence point of the titration.
Web Calculate the molarity of 3000 mL of an acetic acid solution if 2730 mL of 0275 M barium hydroxide was added at the equivalence point. Web Spread the loveThe equivalence point in a titration is a crucial concept in chemistry as it represents the exact moment when the amount of added titrant is. Youll get a detailed solution from a subject matter expert that helps you learn core concepts.
0050 L 6 molL 03 moles of strong acid added thus far. For a 500 mL sample of blood it takes. We want to measure the amount of chemical A in a blood sample using chemical C where A C D.
An HA A 2 buffer controls the pH between the two equivalence points. Web The molarity of the acid is given so the number of moles titrated can be calculated. Determining the Concentration of a Solution Beers Law.
20 22 24 26 28 30 mL NaoH dpHdV Figure 2. Caitlin Bettenay Determining the Concentration. Web In this JC2 webinar we want to learn how to calculate the pH at equivalence point.
If 03 initial. Write the balanced chemical. This is the point at which.
Web When all of a weak acid has been neutralized by strong base the solution is essentially equivalent to a solution of the conjugate base of the weak acid. A student titrated 3000 mL of a weak base. The volume of the titrant addedThis.
Web The pKb of ammonia is 475 at 25C. As shown in part b in Figure 1743 the titration curve for NH3 a weak base is the reverse of the titration curve for. Defining Equivalence Point Equivalence point is a term used to describe the point during an acid-base titration when equal amounts of acid and base have reacted.
When 300 ml of 01M CH3COOH is titrated with 01 M NaOH calculate the.

Why Does The Ph Before The Equivalence Point Of A Titration Depend On The Initial Concentration Only When The Acid Is Strong Chemistry Stack Exchange

30 In Binary How To Convert 30 From Decimal To Binary
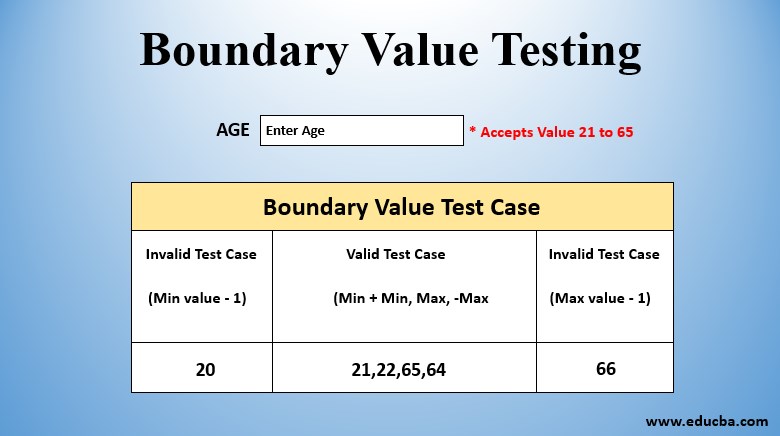
Boundary Value Testing What Is Boundary Value Testing And Examples
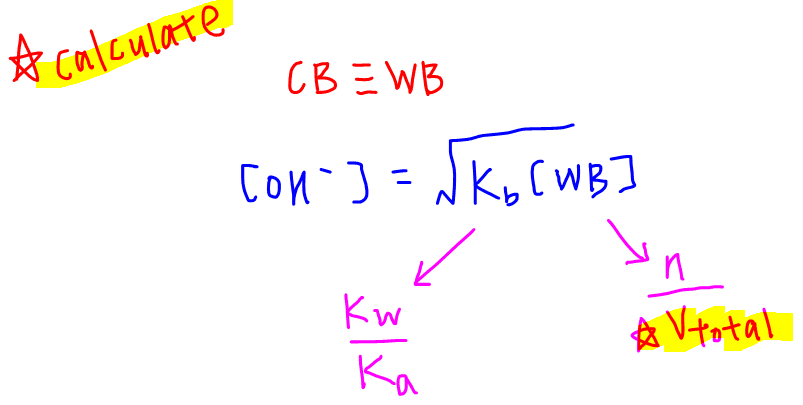
How To Calculate Ph At Equivalence Point
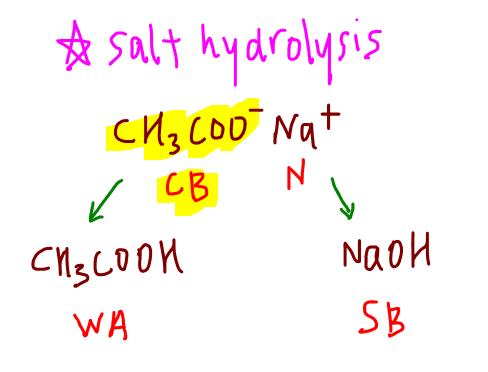
How To Calculate Ph At Equivalence Point
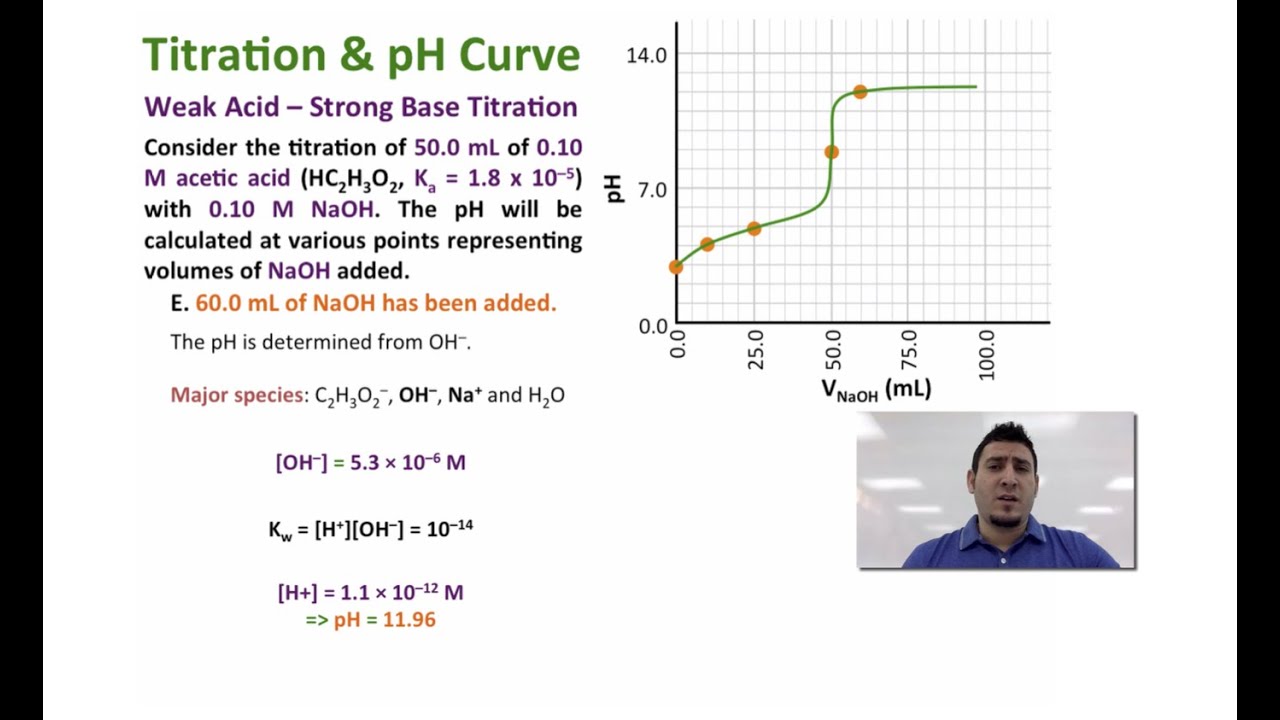
How Do You Calculate The Ph At The Equivalence Point For The Titration Of 190m Methylamine With 190m Hcl The Kb Of Methylamine Is 5 0x10 4 Socratic

How To Find The Half Equivalence Point In A Titration Graph Sciencing

Ph Calculations Involving Titrations Chad S Prep

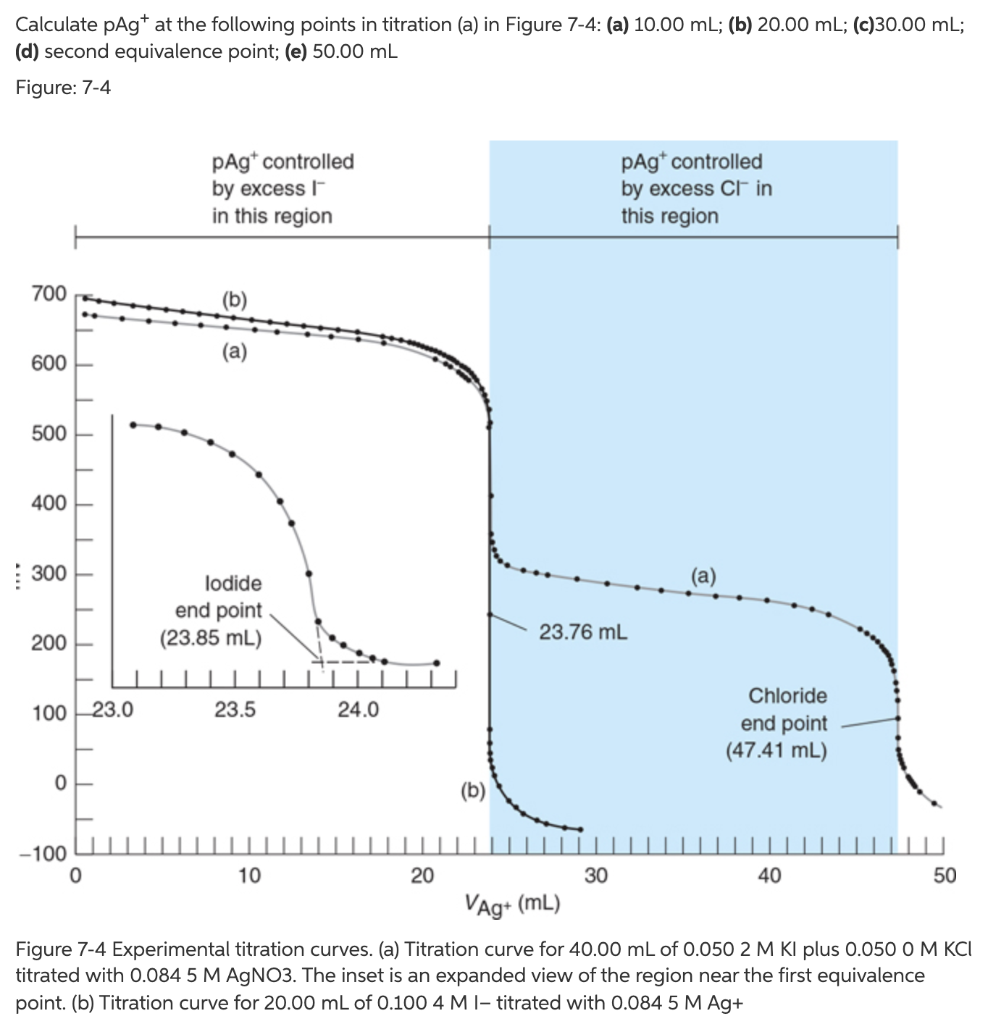
Solved Calculate Pagt At The Following Points In Titration Chegg Com

Equivalence Point Labster
Vegetable Names Explore The List Of 30 Names In English
Social Security Early Retirement 2024 Know Your Bend Points Physician On Fire

See Summary Top Of P 778 In Textbook Ppt Download

Titration Curves Equivalence Point Calculations Chemtalk

What Is Linear Regression Spiceworks Spiceworks

Weak Acid Strong Base Titration Ph At Equivalence Point Youtube
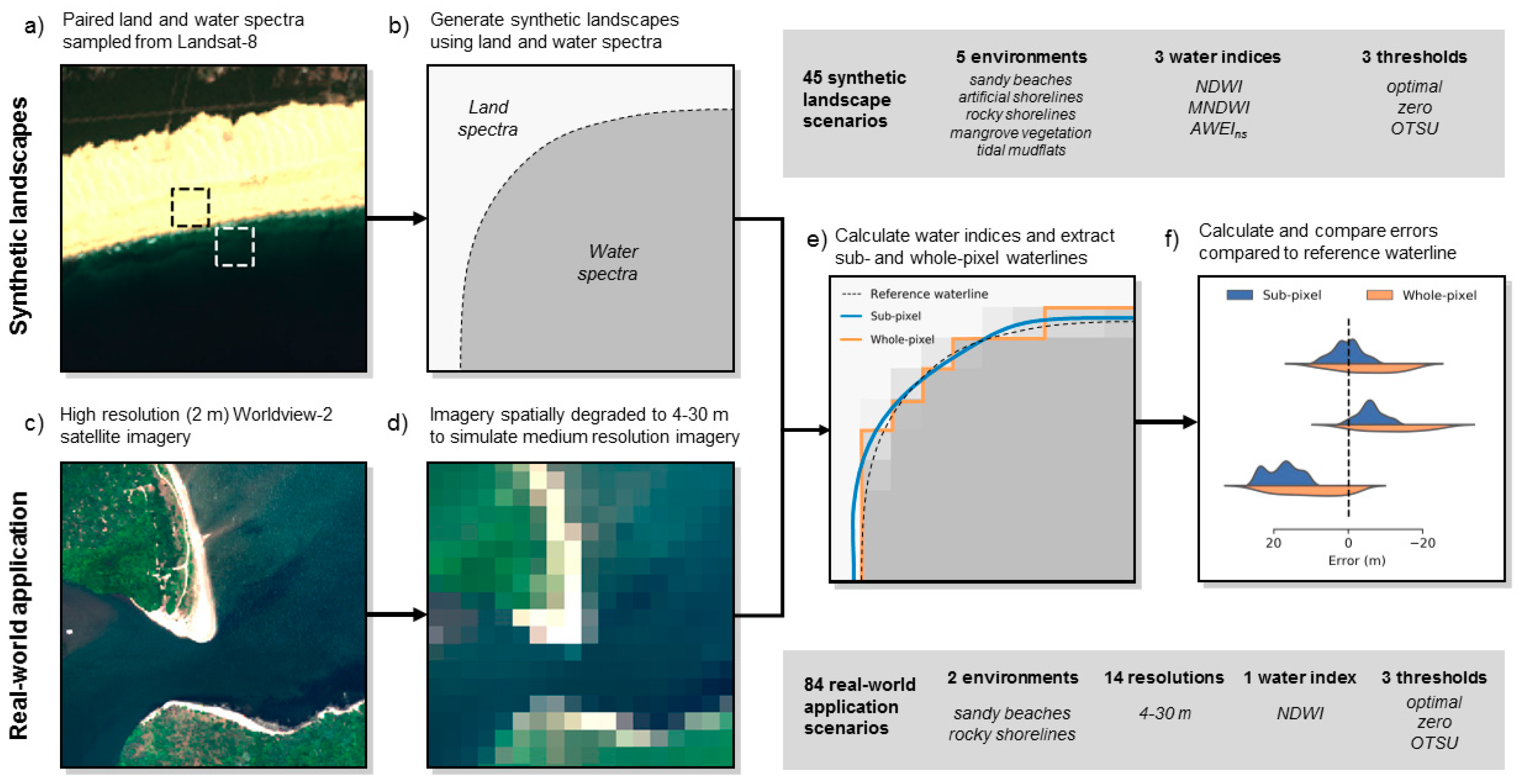
Remote Sensing Free Full Text Sub Pixel Waterline Extraction Characterising Accuracy And Sensitivity To Indices And Spectra